
Kalamassery, Kochi, India - 682022.
Verified 12 yrs of Exp
21
Details verified of Shijo F.✕
 Identity
Identity
 Education
Education
Know how UrbanPro verifies Tutor details
Identity is verified based on matching the details uploaded by the Tutor with government databases.



+5 more
Malayalam Mother Tongue (Native)
English Proficient
Kannada Basic
Hindi Basic
Tamil Basic
![]() Mg university 2014
Mg university 2014
Bachelor of Science (B.Sc.)
![]() Mg university 2016
Mg university 2016
Master of Science (M.Sc.)
 Ministry of human resources department, govt of india 2018
Ministry of human resources department, govt of india 2018
Gate 2018
Kalamassery, Kochi, India - 682022
![]() ID Verified
ID Verified
![]() Education Verified
Education Verified
![]() Phone Verified
Phone Verified
![]() Email Verified
Email Verified
![]() Facebook Verified
Facebook Verified
Report this Profile
Is this listing inaccurate or duplicate? Any other problem?
Please tell us about the problem and we will fix it.
Class Location
![]() Online class via Zoom
Online class via Zoom
![]() Student's Home
Student's Home
![]() Tutor's Home
Tutor's Home
Years of Experience in Class 11 Tuition
12
Board
CBSE
Experience in School or College
12 yr of experience
Subjects taught
Chemistry
Taught in School or College
Yes
Class Location
![]() Online class via Zoom
Online class via Zoom
![]() Student's Home
Student's Home
![]() Tutor's Home
Tutor's Home
Years of Experience in Class 12 Tuition
12
Board
CBSE
Subjects taught
Chemistry
Taught in School or College
No
Class Location
![]() Online class via Zoom
Online class via Zoom
![]() I am willing to Travel
I am willing to Travel
![]() Tutor's Home
Tutor's Home
Years of Experience in UGC NET Exam Coaching classes
10
UGC_NET_Papers
Paper II / Paper III
Subject
Chemistry
Class Location
![]() Online class via Zoom
Online class via Zoom
![]() Student's Home
Student's Home
![]() Tutor's Home
Tutor's Home
Years of Experience in Class 10 Tuition
15
Board
State, CBSE, ICSE
Experience in School or College
5y ears
Subjects taught
Science, Chemistry
Taught in School or College
Yes
Teaching Experience in detail in Class 10 Tuition
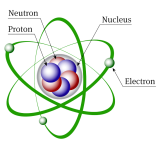
I have done my BSC and msc from the store berchmans college , CHANGANACHERRY. Then I had worked as a tutor in some tutorial s near to my area. Now I am doing my PhD in Cochin University, kalamasserry. As I had 3 years of teaching experience , I have good understanding in chemistry. I can able to take a friendly class for students knowing there weak point. Now a days good job opportunities are getting more and more in the field of chemistry. And moreover now a days students like to build there Carrier in medical and engineer ing field where chemistry is an essential part of there studies. As a result student want to build good understanding from the basic itself. Now a days most of the students face issue in the organic section. And I can able to teach them organic in a better way. I will almost all the topics in there syllabus. I will teach them how to write product by looking at the reagents and basic roles of all the reagents. Elemental idea and basic characteristics are important in the chemistry . The periodic table is an important part of the chemistry. Basically idea about the chemistry will help them a lot in the practical. Once they started learning chemistry everything will be fine for them . Chemistry has a lot of application in there daily life and lot of startups are started in the field of chemistry.
Upcoming Live Classes
5 out of 5 4 reviews
Amarjith v dev
"Deep knowledge in chemistry. Particularly in organic chemistry. Hard working mentality. Good leadership quality. "
Arun
"My first interaction with Shijo was when we were working together. He was always a positive vibe an was a very dedicated and inspiring friend and colleague. He always had a very peculiar way of analyzing and identifying the solutions to problems, whether it will technical or non-technical. He always had that easiness in explaining even the hardest and complicated topics in a very easy and way. While explaining, he relates subjects to our day to day life so that we can understand the concepts clearly and easily. And one great character of Shio was his cool. He was always very mature in handling situations and always gave clear cut advice that was always beneficial for coping difficult situations. He also had hands on experience with apparatus like HPLC, GC, and NMR, so he was able to explain those things clearly from his experience. As a whole he was of a very great support and a good guide for me. "
Sherin Joseph
Periodic Table and Basic Organic Chemistry Classes
"Super class, easy to understand. Maintain better relationship with students. Give notes for study. Any time assistance. "
Jithin Rafi
Periodic Table and Basic Organic Chemistry Classes
"Nice classes. His way of teaching is good. He have well basics in this field. All the very best for his PhD career. "
1. Which school boards of Class 12 do you teach for?
CBSE
2. Have you ever taught in any School or College?
Yes
3. Which classes do you teach?
I teach Class 10 Tuition, Class 11 Tuition, Class 12 Tuition and UGC NET Exam Coaching Classes.
4. Do you provide a demo class?
Yes, I provide a paid demo class.
5. How many years of experience do you have?
I have been teaching for 12 years.
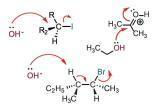
Organic chemistry means how particular reagents will react. Example if we considering acid it will make a proton donor or an electron acceptor. That is when was an acid is added it would acidify the medium...
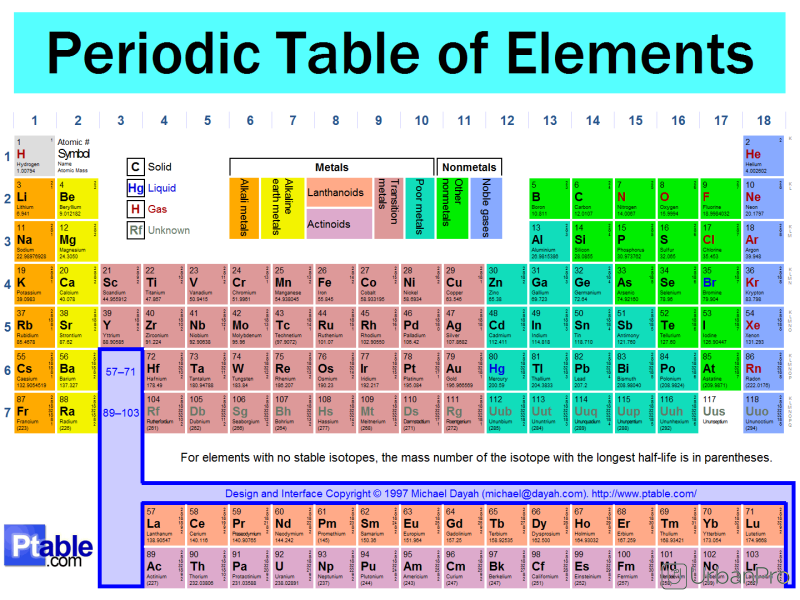
The elements are classified as S P D F blocks based which shells there valance electrons enters. When we comparing the periodic properties Atomic size : As no shells increases size also will increases...
Answered on 05/07/2020
Answered on 05/07/2020
Answered on 05/07/2020
Answered on 05/07/2020 Learn CBSE - Class 11/Chemistry
Answered on 07/01/2019 Learn CBSE - Class 11/Chemistry/Unit 10-s -Block Elements (Alkali and Alkaline Earth Metals)
Class Location
![]() Online class via Zoom
Online class via Zoom
![]() Student's Home
Student's Home
![]() Tutor's Home
Tutor's Home
Years of Experience in Class 11 Tuition
12
Board
CBSE
Experience in School or College
12 yr of experience
Subjects taught
Chemistry
Taught in School or College
Yes
Class Location
![]() Online class via Zoom
Online class via Zoom
![]() Student's Home
Student's Home
![]() Tutor's Home
Tutor's Home
Years of Experience in Class 12 Tuition
12
Board
CBSE
Subjects taught
Chemistry
Taught in School or College
No
Class Location
![]() Online class via Zoom
Online class via Zoom
![]() I am willing to Travel
I am willing to Travel
![]() Tutor's Home
Tutor's Home
Years of Experience in UGC NET Exam Coaching classes
10
UGC_NET_Papers
Paper II / Paper III
Subject
Chemistry
Class Location
![]() Online class via Zoom
Online class via Zoom
![]() Student's Home
Student's Home
![]() Tutor's Home
Tutor's Home
Years of Experience in Class 10 Tuition
15
Board
State, CBSE, ICSE
Experience in School or College
5y ears
Subjects taught
Science, Chemistry
Taught in School or College
Yes
Teaching Experience in detail in Class 10 Tuition
I have done my BSC and msc from the store berchmans college , CHANGANACHERRY. Then I had worked as a tutor in some tutorial s near to my area. Now I am doing my PhD in Cochin University, kalamasserry. As I had 3 years of teaching experience , I have good understanding in chemistry. I can able to take a friendly class for students knowing there weak point. Now a days good job opportunities are getting more and more in the field of chemistry. And moreover now a days students like to build there Carrier in medical and engineer ing field where chemistry is an essential part of there studies. As a result student want to build good understanding from the basic itself. Now a days most of the students face issue in the organic section. And I can able to teach them organic in a better way. I will almost all the topics in there syllabus. I will teach them how to write product by looking at the reagents and basic roles of all the reagents. Elemental idea and basic characteristics are important in the chemistry . The periodic table is an important part of the chemistry. Basically idea about the chemistry will help them a lot in the practical. Once they started learning chemistry everything will be fine for them . Chemistry has a lot of application in there daily life and lot of startups are started in the field of chemistry.
5 out of 5 4 reviews
Amarjith v dev
"Deep knowledge in chemistry. Particularly in organic chemistry. Hard working mentality. Good leadership quality. "
Arun
"My first interaction with Shijo was when we were working together. He was always a positive vibe an was a very dedicated and inspiring friend and colleague. He always had a very peculiar way of analyzing and identifying the solutions to problems, whether it will technical or non-technical. He always had that easiness in explaining even the hardest and complicated topics in a very easy and way. While explaining, he relates subjects to our day to day life so that we can understand the concepts clearly and easily. And one great character of Shio was his cool. He was always very mature in handling situations and always gave clear cut advice that was always beneficial for coping difficult situations. He also had hands on experience with apparatus like HPLC, GC, and NMR, so he was able to explain those things clearly from his experience. As a whole he was of a very great support and a good guide for me. "
Sherin Joseph
Periodic Table and Basic Organic Chemistry Classes
"Super class, easy to understand. Maintain better relationship with students. Give notes for study. Any time assistance. "
Jithin Rafi
Periodic Table and Basic Organic Chemistry Classes
"Nice classes. His way of teaching is good. He have well basics in this field. All the very best for his PhD career. "
Answered on 05/07/2020
Answered on 05/07/2020
Answered on 05/07/2020
Answered on 05/07/2020 Learn CBSE - Class 11/Chemistry
Answered on 07/01/2019 Learn CBSE - Class 11/Chemistry/Unit 10-s -Block Elements (Alkali and Alkaline Earth Metals)
Organic chemistry means how particular reagents will react. Example if we considering acid it will make a proton donor or an electron acceptor. That is when was an acid is added it would acidify the medium...
The elements are classified as S P D F blocks based which shells there valance electrons enters. When we comparing the periodic properties Atomic size : As no shells increases size also will increases...

Share this Profile
Also have a look at
Reply to 's review
Enter your reply*
Your reply has been successfully submitted.
Certified
The Certified badge indicates that the Tutor has received good amount of positive feedback from Students.