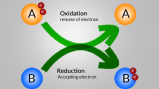
Oxidation-Reduction -one of the most important chapter for chemistry. I will feel you from basic level to standard level . The chapter it's a methodology where in a given reaction one oxidised and one reduced .l simply say that, an atom 'X' when an atom take/gain electron means it reduced(X negative). And when X atom loose electron means it oxidised(X positive)
X+e— →X—
X →X^+ +e—
Now we discuss about classification.
1.Disproportionation reaction.
2.Comproportionation reaction.
#1.DISPROPORTIONATION REACTION :- A radical that is in an intermediate oxidized state in a redox reaction simultaneously oxidised and reduced. It's called as disproportionation reaction.
Eg.
M^+ → M + M—
#2.Comproportionation Reaction:-
two reactants with different oxidation states of the same element react to form a single product with an intermediate oxidation state
Eg:- Ag2+(aq)+Ag(s) → 2Ag+(aq)
where Ag2+ to 2Ag+ it's a Reduction (+2 oxidation state to +1)
And Ag to 2 Ag+ it's a oxidation (zero (0) to +1)
I summarise the key concept of redox chapter. Thank you