Download free printable IIT JEE Worksheets to practice. With thousands of questions available, you can generate as many IIT JEE Worksheets as you want.
Download WorksheetA block of mass 2 kg slides down a frictionless incline of height 5m. At the bottom, its speed is:
5m/s
2.7m/s
3.10m/s
4.14m/s
1. A force of 10 N, displaces a body by 2m in the direction of the force. The work done is:
5j
2.10j
3.20j
4.40j
the ______ is the length of the path travelled by light in vaccum during a time interval of 1/299792458 of a second
kilogram
2.second
3.ampere
4.metre
Identify which of the following properties is extensive .
(a) volume
(b) temperature
(c) boiling point
volume
2.temperature
3.volume and temperature
4.boiling point
Classify each of the following as a compound:
(a) copper
(b) water
(c) nitrogen
(d) sulfur
(e) air
(f) sucrose
(g) a substance composed of molecules each of which contains two iodine atoms
a , b , f , g
2.b , f
3.a,c , d
4.e, f
The smallest particle of an element that can enter into a chemical combination is
element
2.compound
3.H2O
4.atom
If a cake is chosen at random then find the probability that it has four candles?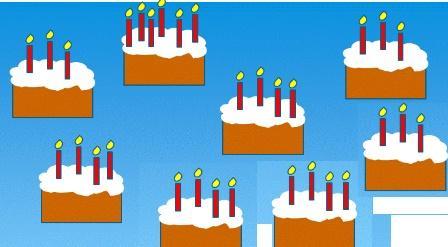
3/8
2.1/2
3.1/8
4.5/8
Which of the physical quantity is a scalar?
Velocity
2.Displacement
3.Acceleration
4.Speed
Total no of Isomeric Structures possible with M.F C7H80 ?
HINT :Each structure has a Benzene ring
which of the following increase with tempearture
physical adsorption
2.chemical adsorption
3.rate of the reaction
4.both A and C
5.0
Worksheets by UrbanPro
A little About Us
Our worksheets are designed to help students explore various topics, practice skills and enrich their subject knowledge, to improve their academic performance. Designed by Experts who have extensive experience and expertise in teaching a subject, these worksheets will improve your child's problem-solving skills and subject knowledge in a fun and interactive manner.
Check out our free customized worksheets across school boards, grades, subjects and levels of subject knowledge. You can download, print and share these worksheets with anyone, anywhere, anytime!
 Worksheet
Worksheet