Your Worksheet is Ready
CBSE - Class 10 Science Metals and Non-metals Worksheet
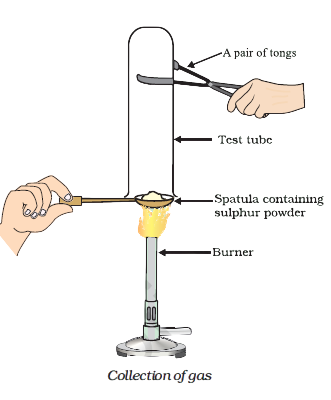
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below. (b) Write a balanced chemical equation for the reaction taking place.
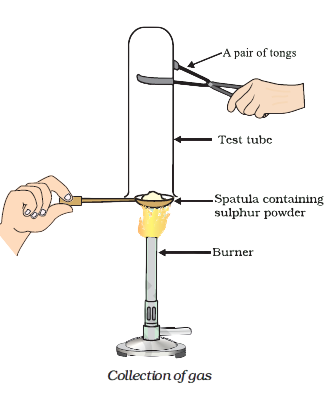
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below. (a) What will be the action of gas on (ii) moist litmus paper?
Which of the following methods is suitable for preventing an iron frying pan from rusting?
Applying grease
B)Applying paint
C)Applying a coating of zinc
D)All of the above.
An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
calcium
B)carbon
C)silicon
D)iron.
Which of the following pairs will give displacement reactions?
$NaCl$ solution and copper metal
B)$MgCl_2$ solution and aluminium metal
C)$FeSO_4$ solution and silver metal
D)$AgNO_3$ solution and copper metal.
Food cans are coated with tin and not with zinc because
zinc is costlier than tin.
B)zinc has a higher melting point than tin.
C)zinc is more reactive than tin.
D)zinc is less reactive than tin.
CBSE - Class 10 Science Metals and Non-metals Worksheet
Answers